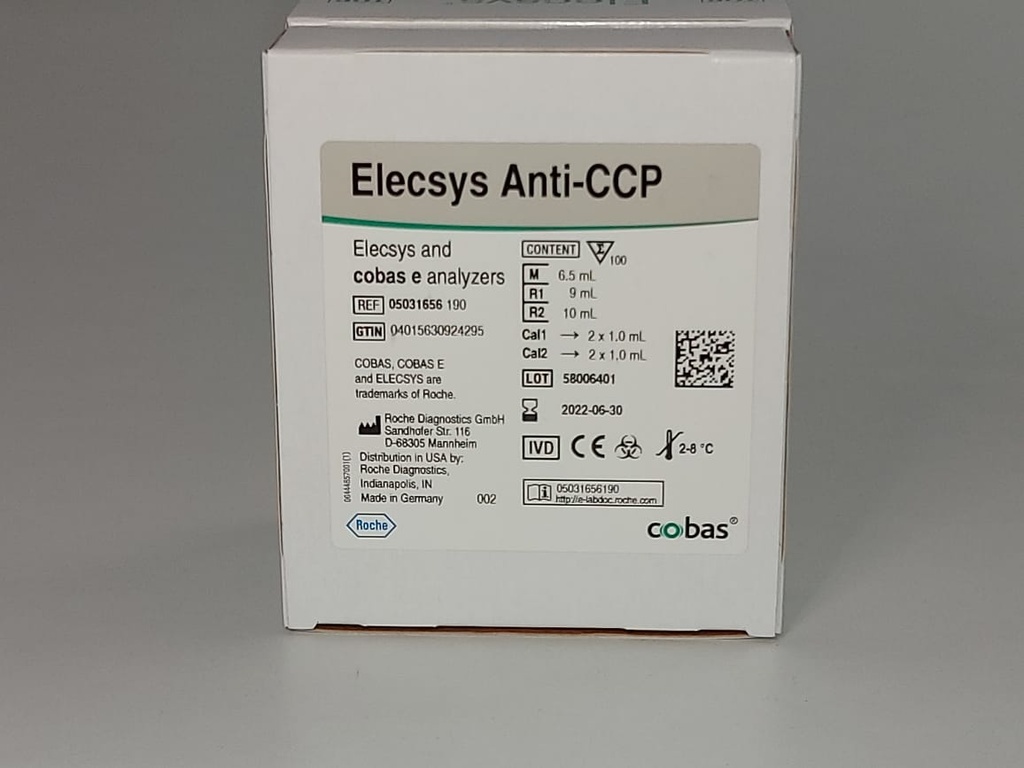
ROCHE ELECSYS Anti-CCP (100 Test)
Barcode: 05031656190 1Product Documents
Category:
System information
For cobas e 411 analyzer: test number 810
For cobas e 601 and cobas e 602 analyzers: Application Code Number 202
Please note
The measured anti‑CCP value of a patient’s sample can vary depending on the testing procedure used. The laboratory finding must therefore always contain a statement on the anti‑CCP assay method used. Anti‑CCP values determined on patient samples by different testing procedures cannot be directly compared with one another and could be the cause of erroneous medical interpretations. Therefore, the results reported by the laboratory to the physician should include: “The following results were obtained with the Elecsys Anti‑CCP assay. Results from assays of other manufacturers cannot be used interchangeably.”
Intended use
Immunoassay for the in vitro semi-quantitative determination of human IgG autoantibodies to cyclic citrullinated peptides in human serum. The results of the assay are intended to be used as an aid in the diagnosis of rheumatoid arthritis in combination with other clinical and laboratory
findings.
The electrochemiluminescence immunoassay “ECLIA” is intended for use on Elecsys and cobas e immunoassay analyzers.

To install this Web App in your iPhone/iPad press ![]() and then Add to Home Screen.
and then Add to Home Screen.